Myopathies are diseases of muscle. Given that mitochondria play essential roles in energy regulation and production, it should come as little surprise that mitochondrial myopathy can arise from mitochondrial dysfunction. Tragically, some myopathies can interfere with the ability to do even basic and simple human moments and tasks. Mitochondrial myopathies also have neurological components. With regard to mitochondria, in addition to playing a role in energy production, defects in mitochondria can affect nerve response and neuromuscular functions sufficient to influence the coordination of muscle movement.
Symptoms and types of mitochondrial myopathy
Most of the mitochondrial-related myopathies are genetic in origin, although some can result from the exposure to drugs (as an example, statins used to control cholesterol production, see below), poor nutrition, or environmental factors, such as herbicides with the toxicological characteristics of inhibitors of mitochondrial function.
The more common symptoms of the mitochondrial-related myopathies are muscle weakness and the inability to control specific muscle groups to perform functions. Those who suffer may have difficulty in swallowing food or have defective speech because of the lack of control in the face and neck muscles. In some cases, there may an inability to control muscle groups Important to eye moments. Exercise intolerance is also a common trait; even simple movements can become exhausting. Regarding the myopathies that are genetic in origin, fortunately, most are very rare in occurrence. They range in severity from progressive weakness to early death. As may be inferred from the descriptions below, most mitochondrial myopathies are expressed early in life. Some examples include:
Kearns-Sayre syndrome – Kearns-Sayre syndrome often starts in the late teens or early twenties. The first manifestations are in the muscles associated with ocular movement eventually leading to paralysis of such muscles. There also is an abnormal buildup of pigmented material, which leads to inflammation and degeneration of the retina. As the disease progresses, cardiac abnormalities, diabetes, and muscular coordination defects become even more apparent. Heart arrhythmia and eventual heart block can result in very early death unless there is intervention with implantation using a cardiac pacemaker.
MERRF (Myoclonic epilepsy with ragged-red fibers) syndrome) – MERRF is rare with an estimated incidence of ~1/400,000. The disorder is characterized by progressive myoclonic epilepsy (involuntary muscle twitching with seizures), which is often apparent at birth. Individuals with MERRF are usually short in stature, suffer hearing loss, and have poor motor skills.
MELAS syndrome – MELAS (Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes) is also characterized by seizures, headaches, and episodes of paralysis. There is often-severe cognitive impairment.
Pharmaceuticals and Myopathies
Some myopathies can also occur from exposure to drugs and toxicants. An example of myopathy that may be drug related is those that potentially evolve or be expressed by the use of statins. Statins are in general very safe, well tolerated and the most efficient drugs for the treatment of elevated cholesterol. However, an adverse effect of statins is myotoxicity. Although the exact pathophysiology of statin-induced myopathies is not fully understood, important mitochondrial cofactors arise from the cholesterol biosynthesis pathway, such as CoQ10. One possibility for a myopathy is a reduction in sufficient COQ10 to maintain normal energy production.
In addition, it has recently been shown that the use of statins is associated with the potential expression of a variety of inflammatory myopathies including polymyositis (chronic inflammation of the muscles), dermatomyositis (inflammation that occurs in both muscle and skin), and necrotizing myopathy (degradation and loss of muscle fibers). With regard to necrotizing myopathy, recent data suggest the serum of some of the patients receiving statins contains an anti-HMGCR antibody. Apparently in addition to pharmacologically inhibiting the first step in cholesterol synthesis, i.e. inhibiting the enzyme, 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR), there is also up-regulation in the expression of autoantibodies to HMGCR, which can further alter not only cholesterol synthesis, but the synthesis of other compounds, such as CoQ10 that are important mitochondrial function. For some patients, the issue can be a very important one, particularly when there is actual muscle damage or loss. For example, anti-HMGCR autoantibodies were recently observed in 6% of patients presenting to the Johns Hopkins Myositis Center. Among patients, ages 50 years and older, over ninety percent had taken statins (Mammen et al. 2011 Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum;63:713-721). This suggests that statins can cause or unmask an immune-mediated myopathy.
Myopathies and environmental factors
Although rare, some signs of mitochondrial myopathy may result from the exposure to environmental factors, such as herbicides. The active principles in some pesticides are compounds that inhibit the mitochondrial oxidative metabolism that leads to ATP production. A good example is rotenone. Rotenone is an odorless chemical that is used as a broad-spectrum insecticide, and pesticides. It occurs naturally in the roots and stems of several plants such as the jicama vine plant. Jícama is often cut into thin wedges and is often used in salads, fresh fruit combos, fruit bars, soups, and other cooked dishes. Although the root may be consumed, the remainder of the jicama plant is poisonous, because of the toxin, rotenone.
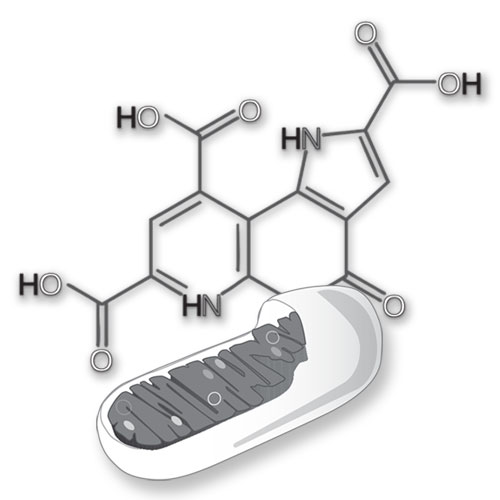
Can PQQ be beneficial in the treatment of certain myopathies?
The answer may be yes in many cases. Mitochondria replicate more often than the cells in which they live. Generally, the faster mitochondria turn over, the better. In other words, it is better to replace mitochondria before too much mitochondrial DNA damage accumulates. Although PQQ stimulates mitochondriogenesis, it may also be assumed that the process involves, in addition, some degree of apoptosis (mitochondrial degradation and replacement). Thus, turnover may be increased and to some degree the DNA in mitochondria may be spared from persisting in a damage form.
Appreciate, however, that these changes are not huge. A change as little as 5 to 15 percent in increased mitochondrial production can be dramatic from the perspective energy metabolism. With regard to caveats, whether PQQ helps or not depends on the nature of the myopathy. There are 600-1000 nuclear genes involved in mitochondrial assembly and in the mitochondrial genome a single circular chromosome that has about 40 genes. This means that in many diseases wherein mitochondria may be involved, there are also numerous possibilities for disease-related mechanisms. Increasing mitochondrial amount may be helpful. However, the increase has the potential of carrying with it an increase in a defective gene product.
Would you like to learn more about PQQ? Check out the article: Pyrroloquinoline Quinone and mTORs

On a diagnostiqué chez moi, une myasthénie bucco-pharyngée puis aprés une sclérose latérale primitive en 2002. Aujourd’hui importants problèmes de douleurs musculaires et des pertes d’équilibre, avec de la faiblesse dans les jambes. PQQ est-il un complément pour mon cas? BONSOIR
Sorry Christian, I think your question is in French, and whether I am right or not whatever it is I do not understand your pyrroloquinoline quinone question. Can you try again in English?
Michael, I copied Christian’s question and then pasted it into a free online translation website and instructed the program to translate from French to English. #1 You have been diagnosed with me, a myasthenia gravis bucco-pharyngee then after a primitive lateral sclerosis in 2002. Today important problems of muscle pain and loss of balance, with the weakness in the legs. PQQ is it an add-in for my case. #2 They diagnosed at home, a myasthénie bucco-pharyngée then aprés a primitive lateral sclerosis in 2002. Today serious problems of muscular pain and the losses of equilibrium, with weakness in legs. Is… Read more »
Thanks John. It appears that Christian’s questions relate to using PQQ to treat disease and my answer here is always going to be that something as serious as muscular dystrophy should be discussed with your doctor. That said, there is no direct scientific proof pyrroloquinoline quinone would help.