Before we talk about quinone structure, let’s talk a little about quinones. Quinones are derived from aromatic compounds, such as benzene and naphthalene. There are thousands of aromatic compounds. Although a thorough discussion of organic chemistry related to quinone structure and aromatic compounds is somewhat beyond the scope of this informational site about pyrroloquinoline quinone, it is important to appreciate some of the following quinone structural characteristics. Electrons within aromatic structures can move from one site to another very rapidly. The arrangement of atoms in aromatic structures makes such compounds very stable from a chemical perspective.
Aromaticity, as a chemical property, results from rings of atoms (usually carbon and other small atoms that are capable of forming stable multiple chemical bonds) being linked by conjugated doubled bonds (…-C=C-C=C-…). The adjacent double bond arrangement (=) allows electrons to resonate (move back and forth and around the ring). Quinones can be structured as a part of this arrangement and occur upon the conversion of an even number of –CH= groups into –C(=O)– groups (with any necessary rearrangement of double bonds). Quinones are conjugated (i.e. they maintain the –[C-H]= or –[C=O]– single-double bond arrangements), but when quinone functions are introduced, their structure can lose other aromatic properties.
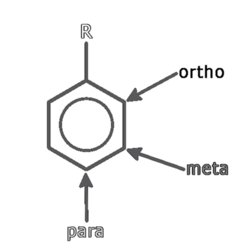
Quinones are commonly named with a prefix that indicates the parent aromatic hydrocarbon (“benzo-” for benzene, “naphtho-” for naphthalene, “anthra-” for anthrocene) and quinone as the suffix. Quinone structure can be arranged in ortho, meta, or para positions around an aromatic ring, as shown in the following example:
Derivatives of quinones are common constituents of biologically relevant molecules. Some examples are vitamins K and E as well as compounds directly involved in oxidative metabolism, such as CoQ10. Some have accessory structures, such as phytyl side chains, which are long carbon-containing lipophylic chains that facilitate association with specific sites in cellular lipid membranes. In nature, quinones act as coloring substances and, similar to CoQ10, as redox (electron shuttling compounds). Many of the antioxidants found in foods are quinones (e.g. flavonoids and flavonoids). Among these compounds are derivatives of quercetin (in fruits and vegetables), resveratrol (in red wine), catechin and epicatechin (in chocolate and tea), and compounds derived from amino acids, such as tyrosine and tryptophan (hydroxytyrosol, 5-hydroxytryptophan, and of course, PQQ).
The ability of quinones to accommodate electrons in novel ways often imparts antioxidant potential. A good example is vitamin E, which inhibits membrane lipid oxidation. In a more general context, oxidation reactions can produce free radicals, which can initiate chain reactions that also damage other cellular components. Antioxidants terminate chain reactions by removing free radical intermediates and inhibit other oxidation reactions. They do this by being oxidized themselves, so antioxidants are also often reducing agents. Chemical stability, due to aromatic character, and rapid election movement (resonance), and the ability to carry out redox reactions contribute to making these compounds nutritionally important and often “protective” as antioxidants or redox facilitators.
With regard to biofactors, such as PQQ, resveratrol, and hydroxytyrosol that can function to stimulate given steps in mitochondriogenesis, it remains unclear exactly what the quinone function does. It may be important in interacting with redox-sensitive sites on specific receptor proteins important to mitochondrial cell signaling pathways. It is obvious that the structure of the compound that is quinone-containing is also important regarding how these compounds get recognized and used in biological systems.
More broadly, one may also ask whether a given vitamin or biofactor containing a quinone moiety provide multiple functions? For some quinones, the answer is definitely yes. Blood plasma containing trace amounts of pyrroloquinoline quinone has antioxidant activity in chemical assays, in contrast to plasma devoid of PQQ. In some bacteria, PQQ acts as a redox cofactor for enzymes called dehydrogenases. An example is the oxidation of substances containing alcohol groups (–CH2OH) to aldehydes (–CHO). Such reactions involving methoxatin have not been observed in animal tissues; however, PQQ can be important to cell signaling. In animal cells, vitamin K is a redox cofactor in reactions important to blood clotting and bone formations, whereas vitamin E acts as an antioxidant and possibility a cell signal molecule. Certain quinone containing plants seem to be involved in the conversion of light energy to chemical energy and are antioxidants when consumed by animals as a part of their diets.
In summary, nature uses the quinone structure in quinone-structured compounds in a number of diverse pathways and functions. The aromatic nature of the many of these compounds imparts chemical stability, which is exactly what is needed if they have to function in an oxidative environment and efficiently engage in repeated reduction-oxidation reactions or as anti-oxidants.
